
(redirected from Atomic oxygen)
- From about 1900 until 1961, oxygen was used as the reference standard, with an assigned value of 16. The unit of atomic mass was thereby defined as 1/16 the mass of an oxygen atom.
- Gaseous chemical element, symbol: O, atomic number: 8 and atomic weight 15,9994. It’s of great interest because it’s the essential element in the respiratory processes of most of the living cells and in combustion processes.
- Atomic and molecular oxygen are chemical species derived from the chemical element, oxygen which has the atomic number 8. The difference between atomic oxygen and molecular oxygen is that atomic oxygen is highly reactive and does not exist in the atmosphere as it is whereas molecular oxygen is less reactive and exists in the atmosphere as it is.
- Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The chemical symbol for Oxygen is O. Atomic mass of Oxygen is 15.9994 u.
Also found in: Dictionary, Thesaurus, Medical, Acronyms.
The chemical symbol for oxygen is O, but it is frequently written as O2. This is because when oxygen is not chemically combined with anything else, it bonds to another oxygen atom, making an oxygen molecule. Oxygen is a diatomic molecule, and that.
oxygen,
gaseous chemical element; symbol O; at. no. 8; interval in which at. wt. ranges 15.99903–15.99977; m.p. −218.4°C;; b.p. −182.962°C;; density 1.429 grams per liter at STP; valence −2. The existence and properties of oxygen had been noted by many scientists before the announcement of its isolation by Priestley in 1774. Scheele had also succeeded in preparing oxygen from a number of substances, but publication of his findings was delayed until after that of Priestley's. As a result, Priestley and Scheele are credited with the discovery of the element independently. The fact that the gas is a component of the atmosphere was finally and definitely established by Lavoisier a few years later. In 1929, W. F. Giaque and H. L. Johnston announced the discovery of two isotopes of oxygen, of mass numbers 17 and 18.Properties and Compounds
Oxygen is a colorless, odorless, tasteless gas; it is the first member of Group 16 of the periodic tableperiodic table,
chart of the elements arranged according to the periodic law discovered by Dmitri I. Mendeleev and revised by Henry G. J. Moseley. In the periodic table the elements are arranged in columns and rows according to increasing atomic number (see the table entitled
...Click the link for more information.. It is denser than air and only slightly soluble in water. A poor conductor of heat and electricity, oxygen supports combustion but does not burn. Normal atmospheric oxygen is a diatomic gas (O2) with molecular weight 31.9988. Ozoneozone
, an allotropic form of the chemical element oxygen (see allotropy). Pure ozone is an unstable, faintly bluish gas with a characteristic fresh, penetrating odor. The gas has a density of 2.144 grams per liter at STP.
...Click the link for more information. is a highly reactive triatomic (O3) allotrope of oxygen (see allotropyallotropy
[Gr.,=other form]. A chemical element is said to exhibit allotropy when it occurs in two or more forms in the same physical state; the forms are called allotropes.
...Click the link for more information.). When cooled below its boiling point oxygen becomes a pale blue liquid; when cooled still further the liquid solidifies, retaining its color. Oxygen is paramagnetic in its solid, liquid, and gaseous forms. Although eight isotopes of oxygen are known, atmospheric oxygen is a mixture of the three isotopes with mass numbers 16, 17, and 18.
Oxygen is extremely active chemically, forming compounds with almost all of the elements except the inert gases. Oxygen unites directly with a number of other elements to form oxidesoxide,
chemical compound containing oxygen and one other chemical element. Oxides are widely and abundantly distributed in nature. Water is the oxide of hydrogen. Silicon dioxide is the major component of sand and quartz.
...Click the link for more information.. It is a constituent of many acids and of hydroxides, carbohydrates, proteins, fats and oils, alcohols, cellulose, and numerous other compounds such as the carbonates, chlorates, nitrates and nitrites, phosphates and phosphites, and sulphates and sulphites.
TREMEC TR-6060™ Transmission 6-Speed RWD Manual Transmission The TREMEC TR-6060 six-speed manual transmission is found in high-performance flagship vehicles. Along with the capability to stand up to some of the highest torque engines in the industry, the TR-6060 delivers great overall shift feel with short throws and smooth gear synchronization. TREMEC TR-6060™ Transmission 6-Speed RWD Manual Transmission The TREMEC TR-6060 six-speed manual transmission is found in high-performance flagship vehicles. Along with the capability to stand up to some of the highest-output V-8 engines in the industry, the TR-6060 delivers great overall shift feel with short throws, and smooth gear. Product Description Tremec TR6060 1st or 2nd Gear Hybrid Synchronizer Blocker Ring Kit Upgraded. 1-2 HYBRID SYNCHRONIZER RING SET FOR 6060 TRANSMISSIONS. THIS HYBRID SET IS AN UPGRADE FROM THE ORIGINAL ALL SINTERED METAL. Tremec tr6060.
The common reaction in which it unites with another substance is called oxidation (see oxidation and reductionoxidation and reduction,
complementary chemical reactions characterized by the loss or gain, respectively, of one or more electrons by an atom or molecule. Originally the term oxidation
...Click the link for more information.). The burning of substances in air is rapid oxidation or combustioncombustion,
rapid chemical reaction of two or more substances with a characteristic liberation of heat and light; it is commonly called burning. The burning of a fuel (e.g., wood, coal, oil, or natural gas) in air is a familiar example of combustion.
...Click the link for more information.. The respirationrespiration,
process by which an organism exchanges gases with its environment. The term now refers to the overall process by which oxygen is abstracted from air and is transported to the cells for the oxidation of organic molecules while carbon dioxide (CO2
...Click the link for more information. of animals and plants is a form of oxidation essential to the liberation of the energy stored in such food materials as carbohydrates and fats. The rusting of iron and the corrosion of many metals results from the action of the oxygen in the air.
Natural Occurrence and Preparation
Oxygen is the most abundant element on earth, constituting about half of the total material of its surface. Most of this oxygen is combined in the form of silicates, oxides and water. It makes up about 90% of water, two thirds of the human body and one fifth by volume of air. It is found in the sun, and has a role in the stellar carbon cycle (see nucleosynthesisnucleosynthesis
or nucleogenesis,
in astronomy, production of all the chemical elements from the simplest element, hydrogen, by thermonuclear reactions within stars, supernovas, and in the big bang at the beginning of the universe (see nucleus; nuclear energy).
...Click the link for more information.). Oxygen is prepared for commercial use by the liquefaction and fractional distillation of air and more expensively by the electrolysis of water; it is stored and transported under high pressure in steel cylinders. It can also be obtained by heating certain of its compounds, such as barium peroxide, potassium chlorate, and the red oxide of mercury.
Uses
Oxygen is of great importance in the chemical and the iron and steel industries. Its major use is in steel production, for example in the Bessemer processBessemer process
[for Sir Henry Bessemer], industrial process for the manufacture of steel from molten pig iron. The principle involved is that of oxidation of the impurities in the iron by the oxygen of air that is blown through the molten iron; the heat of oxidation raises the
...Click the link for more information.. The oxyacetylene torchoxyacetylene torch
, tool that mixes and burns oxygen and acetylene to produce an extremely hot flame. This torch can be used for cutting steel and for welding iron and various other metals. The temperature of the flame can reach as high as 6,300°F; (3,480°C;).
...Click the link for more information. is another important industrial application. Oxygen is utilized in medicine in the treatment of respiratory diseases and is mixed with other gases for respiration in submarines, high-flying aircraft, and spacecraft. Liquid oxygen is used as an oxidizer in the fuel systems of large rockets.
Oxygen was formerly the official standard for the atomic weightsatomic weight,
mean (weighted average) of the masses of all the naturally occurring isotopes of a chemical element, as contrasted with atomic mass, which is the mass of any individual isotope. Although the first atomic weights were calculated at the beginning of the 19th cent.
...Click the link for more information. of elements. The chemists used natural oxygen, a mixture of three isotopes, to which the value of 16 was assigned while the physicists assigned the value of 16 specifically to the oxygen isotope 16. In 1961 carbon-12 replaced oxygen as the standard.
Oxygen
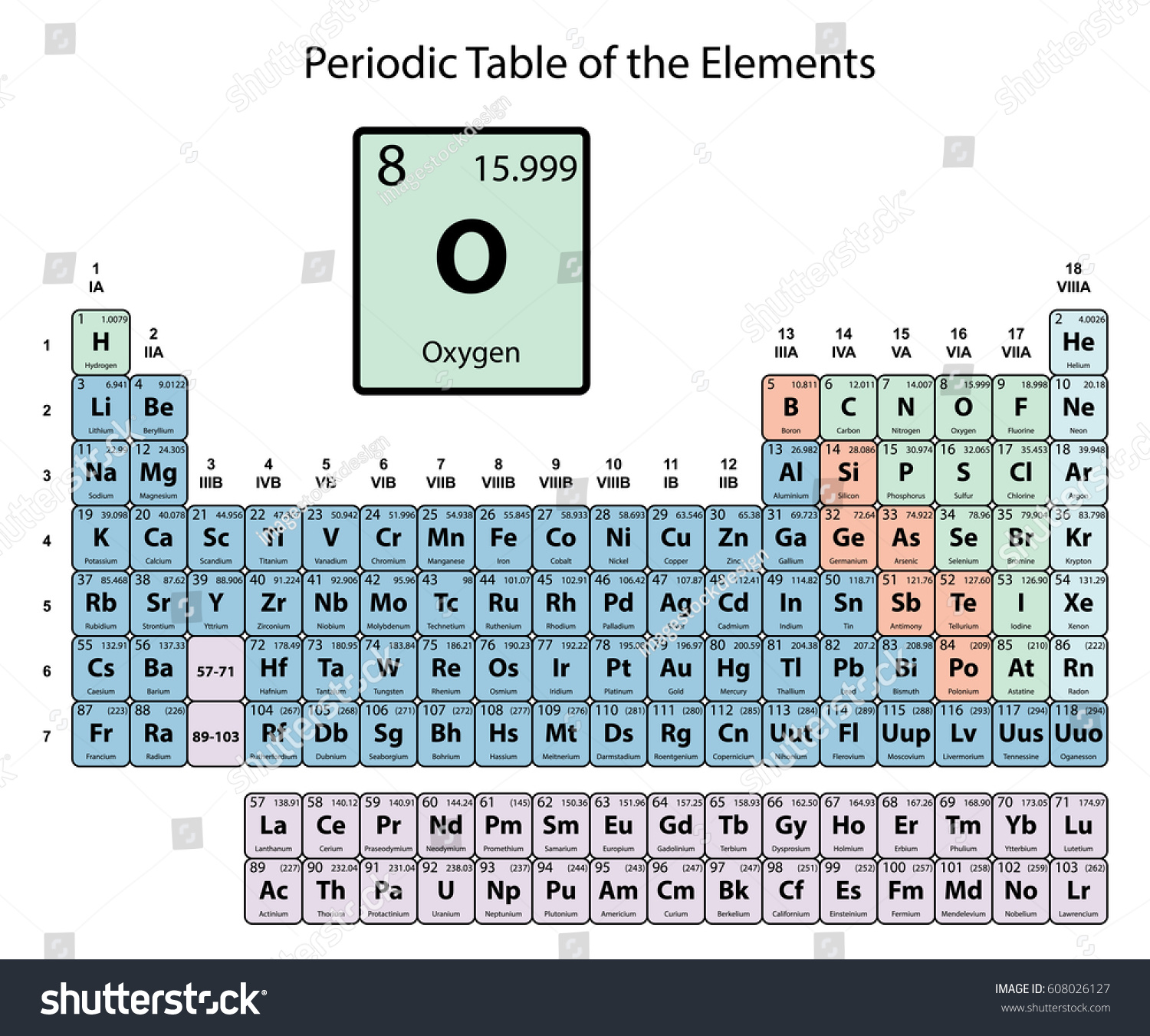
O, a chemical element of group VI of Mendeleev’s periodic system. Atomic number, 8; atomic mass, 15.9994. Under normal conditions, oxygen is a colorless, tasteless, and odorless gas. It would be difficult to name another element that plays as important a role on earth as oxygen.
History. The processes of combustion and respiration have long attracted the attention of scientists. The first hints that not all of the air but only its “active” part supports combustion were found in Chinese eighth-century manuscripts. Much later, Leonardo da Vinci (1452–1519) regarded air as a mixture of two gases, of which only one is consumed during cumbustion and respiration. The ultimate discovery of the two principal constituents of air, nitrogen and oxygen, a discovery of epochal importance in science, was made only in the late 18th century. Oxygen was prepared almost simultaneously by K. Scheele (1769–70) by calcination of nitrates (KNO3, NaNO3), manganese dioxide (MnO2), and other substances and by J. Priestley (1774) by the heating of red lead oxide, Pb3O4, and mercuric oxide, HgO. D. Rutherford discovered nitrogen in 1772. A. Lavoisier, performing a quantitative analysis of air, found that it “consists of two [gases] of different and, so to speak opposite character,” namely of oxygen and nitrogen. On the basis of extensive experimental studies, he correctly interpreted combustion and respiration as processes of the interaction of various substances and oxygen. Since oxygen in a constituent of acids, Lavoisier called it oxygène, that is, “acid former” (from the Greek oxys [acidic]) and gennao [I generate]; hence the Russian name kislorod, from kislyi [acidic] and rodif [to generate].

Distribution in nature. Oxygen is the most widespread element on earth. Warhammer 40,000: dawn of war ii download. Combined oxygen constitutes about six-sevenths of the earth’s hydrosphere (85.82 percent by weight) and almost one-half of the lithosphere (47 percent by weight). Only in the atmosphere, where oxygen is present in the free state, is it the second most abundant element (23.15 percent by weight), after nitrogen.
Oxygen is also in first place in the number of minerals formed by it (1,364). The most widespread minerals containing oxygen are silicates (feldspars, micas), quartz, iron oxides, carbonates, and sulfates. Living organisms on the average contain about 70 percent oxygen; it is a constituent of most of the important organic compounds (proteins, fats, carbohydrates) and a component of the inorganic constituents of the skeleton. The role of free oxygen is especially important in biochemical and physiological processes, particularly in respiration. With the exception of some anaerobic microorganisms, all animals and plants receive the energy required by life processes from the biological oxidation of various substances with oxygen.
The entire mass of the free oxygen on earth originated from and is being maintained by the life activities of green plants, which evolve oxygen in the course of photosynthesis, on land and in the oceans. Photosynthesis and the dominance of free oxygen on the earth’s surface give rise to strongly oxidizing conditions. Conversely, reducing media are formed where oxygen is absent, as in magma, in deep levels of subterranean water, in the silt of the oceans and lakes, and in marshes. Reduction-oxidation processes with the participation of oxygen determine the concentration of many elements and the formation of such mineral deposits as coal, oil, sulfur, iron, and copper ores. The oxygen cycle is also changed by the economic activities of man. In some industrial countries more oxygen is consumed during fuel combustion than the amount evolved by the photosynthesis of plants. The annual oxygen consumption for combustion of fuels on the earth is about 9 X 199 tons.
Isotopes, atoms, and molecules. Oxygen has three stable isotopes—16O, 17O, and 18O—the average content of which constitutes, respectively, 99.759 percent, 0.037 percent, and 0.204 percent of the total number of oxygen atoms on the earth. The predominance of the lightest isotope 16O in the isotope mixture is due to the 16O nucleus consisting of eight protons and eight neutrons. The theory of the atomic nucleus indicates that such nuclei are particularly stable.
In accordance with oxygen’s position in Mendeleev’s periodic system of elements, the electrons of the oxygen atom are arranged in two shells: two in the inner shell and six in the outer shell (configuration Is22s22p4). Since the outer shell is not filled and the ionization potential and the electron affinity are 12.61 and 1.46 eV, respectively, the oxygen atom usually acquires electrons in the course of formation of chemical compounds and has a negative effective charge. Conversely, rare are the compounds in which electrons are torn away (or more precisely, pulled away) from the oxygen atom (as in F2O and F2O2). In the past, proceeding solely from the position of oxygen in the periodic system, a negative charge ( — 2) was ascribed to the oxygen atom. However, experimental data have indicated that the ion O2- does not exist either in the free state or in compounds and that the effective negative charge of the oxygen atom practically never exceeds unity.
Under ordinary conditions, the oxygen molecule is diatomic (O2). A triatomic molecule, ozone (O3), is formed in a silent electric discharge; small amounts of tetratomic molecules (O4) have been detected at high pressures. The electronic structure of O2 is of great theoretical interest. The molecule has two unpaired electrons in the ground state; the usual “classical” structural formula O ⇄ O with two two-electron bonds is inapplicable to it. An exhaustive explanation of this fact is provided by the theory of molecular orbitals.
The ionization energy of the oxygen molecule (O2 — e → O+2) constitutes 12.2 eV, and the electron affinity (O2 + e → O-2)> 0.94 eV. The dissociation of molecular oxygen into atoms is negligible at ordinary temperatures, becoming noticeable only at 1500°C; at 5000°C the oxygen molecules are almost completely dissociated into atoms.
Physical properties. Oxygen is a colorless gas that liquifies at — 182.9°C and normal pressure to a pale blue liquid, which, in turn, solidifies at — 218.7°C forming blue crystals. The density of gaseous oxygen (at 0°C and normal pressure) is 1.42897 g/l. The critical temperature of oxygen is fairly low (tCrit = -118.84°C), that is, lower than that of Cl2, CO2, SO2, and some other gases; Pcrit = 4.97 meganewtons per square meter (49.71 atm). The heat conductivity (at 0°C) is 23.86 X 10-3 W/(m.°K), or 57 X 10-6 cal/(sec · cm.°C). The molar heat capacities (at 0°C) are Cp = 28.9 and Cv = 20.5 in joules (mole·°K) and Cp = 6.99 and Cv = 4.98 in cal/(mole· °C), respectively; CP/CV = 1.403. The dielectric permeability of gaseous oxygen is 1.000547 (at 0°C), and of liquid oxygen 1.491. The viscosity is 189 millipoises (at 0°C). Oxygen is poorly soluble in water: at 20°C and 1 atm, 0.031 m3 dissolves in 1 m3 of water; 0.049 m3 dissolves at 0°C. Platinum black and activated charcoal are efficient solid absorbents for oxygen.
Chemical properties. Oxygen forms chemical compounds with all other elements except the light inert gases. Being the most active nonmetal (after fluorine), oxygen interacts directly with most elements. The only exceptions are the heavy inert gases, the halogens, gold, and platinum; their compounds with oxygen are obtained by indirect methods. Almost all of the reactions involving oxygen are exothermic oxidation reactions, that is, accompanied by the evolution of heat. Oxygen reacts with hydrogen at ordinary temperatures very slowly, whereas this reaction proceeds explosively above 550°C: 2H2 + O2 = 2H2O. Oxygen reacts with sulfur, carbon, nitrogen, and phosphorus very slowly under ordinary conditions. The reaction rate increases with increasing temperature until at an ignition temperature characteristic for each element combustion occurs. The reaction of oxygen with nitrogen is endothermic because of the particular stability of the N2 molecule and becomes noticeable only above 1200°C or in an electric discharge: N2 + O2 = 2NO. Oxygen actively oxidizes almost all metals and, with particular ease, the alkali and alkaline-earth metals. The reactivity of a metal with oxygen depends on many factors, such as the condition of the metal surface, the degree of subdivision, and the presence of impurities.
The role of water is of particular importance in the interaction of substances with oxygen. For example, such an active metal as potassium does not react at all with oxygen that is completely devoid of moisture, but it ignites in oxygen at room temperature in the presence of the minutest quantities of water vapor. It has been calculated that the annual loss owing to corrosion amounts to as much as 10 percent of the entire metal production.
Oxides of some metals form peroxides by the addition of oxygen. The resulting compounds contain two or more oxygen atoms bonded to each other. Thus, the peroxides Na2O2 and BaO2 contain the peroxide ion O22-, the superoxides NaO2 and KO2 contain the ion O2-, and the ozonides NaO3, KO3, RbO3, and CsO3 contain the ion O3-. Oxygen reacts exothermally with numerous complex substances. Thus, ammonia burns in oxygen in the absence of catalysts, the reaction proceeding according to the equation 4NH3 X 3O2 = 2N2 X 6H2O. Oxidation of ammonia with oxygen in the presence of catalysts yields NO (this process is used in the production of nitric acid). Of particular importance is the combustion of hydrocarbons (natural gas, gasoline, kerosene), which constitutes the most important heat source for consumers and industry; for example, CH4 + 2O2 = CO2 + 2H2O. The reaction of hydrocarbons with oxygen forms the basis of many important industrial processes, such as the reforming of methane, which is used for the production of hydrogen: 2CH4 + O2 + 2H2O = 2CO2 + 6H2. Many organic compounds (hydrocarbons with double and triple bonds, aldehydes, phenols, turpentine, drying oils) vigorously add oxygen. The oxidation of nutrients with oxygen in the cells serves as a source of energy for living organisms.
Preparation. There are three basic methods for preparing oxygen: chemical, electrolytic (electrolysis of water), and physical (separation of air).
The chemical method was discovered first. Oxygen may be prepared, for example, from potassium chlorate, KClO3, which decomposes on heating with evolution of O2 in amounts of 0.27 m3 per 1 kg of the salt. Barium oxide, BaO, absorbs oxygen at first, when heated up to 540°C, to give the peroxide BaO2, which decomposes on further heating to 870°C with the evolution of pure oxygen. It may be also obtained from KMnO4, Ca2PbO4, K2Cr2O7, and other substances by heating in the presence of catalysts. The chemical method of preparing oxygen is inefficient and expensive and is used only in laboratory practice.
The electrolytic method consists of passing a direct electric current through water containing a solution of sodium hydroxide, NaOH, to increase its conductivity. In this case, water is decomposed into oxygen and hydrogen. The oxygen is collected at the positive electrode of the electrolysis unit, and the hydrogen at the negative electrode. Oxygen is obtained in this method as a byproduct of hydrogen manufacture. The expenditure of 12–15 kW-hr of electric energy is required for the production of 2 m3 of hydrogen and 1 m3 of oxygen.
The principal method for the production of oxygen in modern technology is the separation of air. To separate air in its normal gaseous state is very difficult and, therefore it is first liquified and then separated into components. This method for the production of oxygen is known as the low-temperature method of air separation. The air is first compressed with a compressor and then, after passing through heat exchangers, expanded in an expansion turbine or through a nozzle; this leads to its cooling to 93°K (—180°C) and conversion to liquid air. Further separation of liquid air, consisting primarily of liquid nitrogen and liquid oxygen, is based on the boiling point differences of its components (the boiling point of O2 is 90.18°K [-182.9°C], and that of N2, 77.36°K [-195.8°C]). Gradual evaporation of liquid air leads, at first, to the evaporation of mainly nitrogen, and the remaining liquid becomes ever more enriched in oxygen. Continuous repetition of this process on the rectification plates of the air-separator columns yields liquid oxygen of the required purity (concentration).
The smallest (capacity of several liters) and largest oxygen air-separation units (35,000 m3/hr of oxygen) in the world have been constructed in the USSR. The units are used in the production of industrial oxygen with a concentration of 95–98.5 percent, technical-grade oxygen with a concentration of 99.2–99.9, and the higher purity oxygen used in medicine. The product may be gaseous or liquified oxygen. The expenditure of electric energy ranges from 0.41 to 1.6 kW-hr/m3.
It is also possible to prepare oxygen by separating air using the method of selective permeation (diffusion) through membrane barriers. Air under pressure is passed through barriers made of fluorocarbons, glass, or plastics, the structural lattices of which are capable of allowing some components to pass through while retaining others. This method of oxygen production has been used to date (1973) only in laboratories.
Gaseous oxygen is stored and transported in steel cylinders and receivers at pressures of 15 and 42 meganewtons per square meter (corresponding to 150 and 420 bars, or 150 and 420 atm, respectively). Liquid oxygen is stored and shipped in metal Dewar vessels or in special tanks. Special pipelines are also used for transporting liquid and gaseous oxygen. Oxygen cylinders are painted light blue and are labeled “oxygen” in black.
Uses. Technical-grade oxygen is used in the gaseous flame working of metals, in welding, in oxygen cutting, in case hardening, in metallization, and in other processes, as well as in aviation, in underwater navigation, and elsewhere. Industrial oxygen is used in the chemical industry for the production of such products as synthetic liquid fuels, lubricating oils, nitric and sulfuric acids, methanol, ammonia, ammonia fertilizers, and peroxides of metals. Liquid oxygen is used in working with explosives, in rocket engines, and in laboratory applications as a cooling agent.
Pure cylinder oxygen is used for breathing at high altitudes, during space flights, and in underwater navigation. In medicine, oxygen is administered intramuscularly and to aid the respiration of the gravely ill; it is also used in oxygen, water, and air baths (in oxygen tents).
V. L. VASILEVSKII I. P. VISHNEV, and A. I. PEREL’MAN
Oxygen is widely used in metallurgy for the enhancement of various pyrometallurgical processes. A full or partial replacement of air fed to metallurgical aggregates with oxygen has changed the chemism of processes, their heat-engineering parameters, and engineering economics indexes. Oxygen blasting has made it possible to reduce heat losses with outgoing gases, which in air blasting largely consisted of nitrogen. Without participating significantly in the chemical processes, nitrogen retarded the reactions by lowering the concentration of the active reagents of the oxidation-reduction medium. Blasting with oxygen lowers the fuel consumption and improves the quality of the metal; new types of production becomes possible in metallurgical aggregates (for example, of slags and gases, which are unusual in composition for a given process and which find special industrial uses).
The first experiments in the use of blast enriched with oxygen in blast-furnace production for the smelting of cast iron for steel production and of ferromanganese were performed simultaneously in the USSR and Germany in 1932–33. The increased oxygen content in the blast of blast furnaces is accompanied by a considerable reduction in the consumption of the oxygen and the simultaneous increase of the carbon monoxide content of the blast-furnace gases and its heat of combustion. Enrichment of the blast with oxygen has made it possible to increase the productivity of the blast furnace and, together with the gaseous or liquid fuel fed to the furnace, leads to a decrease of the coke consumption. In this case, for each additional percent of oxygen in the blast, the productivity increases by about 2.5 percent, and the consumption of coke decreases by 1 percent.
Oxygen in open-hearth production in the USSR was first used to increase fuel combustion efficiency (the first industrial uses of oxygen for this purpose were at the Serp i Molot and the Krasnoe Sormovo plants in 1932–33). The first blasting of oxygen into the bath was begun in 1933 to oxidize the impurities in the final stages. An increase in blasting intensity of the melt to 1 m3/ton/hr leads to a 5–10 percent increase in furnace productivity and a 4–5 percent decrease in fuel consumption. However, metal losses increase during the blasting. An oxygen consumption of up to 10 m3ton/hr leads to an insignificant decrease in the yield of steel (up to 1 percent). Oxygen is finding increasing use in open-hearth production. Thus, while 52.1 percent of steel was produced in 1965 using oxygen in open-hearth furnaces, 71 percent was produced in 1971.

Experiments in the use of oxygen in electric-arc steel-smelting furnaces in the USSR were begun in 1946 at the Elektrostal’ Plant. Adoption of oxygen blasting has made it possible to increase furnace productivity by 25–30 percent, to decrease the specific consumption of electric energy by 20–30 percent, to improve the quality of steel, and to reduce the consumption of electrodes and certain alloys in short supply. Feeding oxygen into electric furnaces proved to be especially effective in the production of stainless steel of low carbon content, which is difficult to manufacture owing to the carburizing effect of the electrodes. The quantity of electric steel being produced in the USSR using oxygen is continuously increasing and in 1970 constituted 74.6 percent of the overall steel production.
In cupola furnace smelting, oxygen-enriched blast is mainly used to superheat cast iron, which is necessary in the production of high-quality melts, particularly highly alloyed ones (with silicon, chromium, and other metals). Depending on the extent of oxygen enrichment of the cupola furnace, the fuel consumption is reduced by 30–50 percent, the sulfur content of the metal is reduced by 30–40 percent, the productivity of the cupola furnace is increased by 80–100 percent, and the temperature of the resulting cast iron is substantially increased (up to 1500°C).
The use of oxygen in nonferrous metallurgy was begun somewhat later than in ferrous metallurgy. The blasting air enriched in oxygen is used in the conversion of mattes, in the slag sublimation process, in the rotary-kiln process, in the agglomeration process, and in the reverberatory smelting of copper concentrates. Oxygen blasting in lead, nickel, and copper production has increased the efficiency of shaft smelting by reducing coke consumption by 10–20 percent, increasing the output by 15–20 percent, and reducing the flux quantity in some cases by a factor of 2–3. An increase of up to 30 percent in the oxygen content of the air blast used in the smelting of zinc sulfide concentrates has led to a 70 percent increase in production and a 30-percent reduction in the volume of outgoing gases. New, highly efficient processes are being developed for the smelting of sulfide materials using pure oxygen: smelting in an oxygen jet, conversion of mattes in vertical converters, and smelting in molten baths.
REFERENCES
Chugaev, L. A. Otkrytie kisloroda i teoriia goreniia v sviazi s filosofskimi ucheniiami drevnego mira. Izbr. trudy, vol. 3. Moscow, 1962. Page 350.Cotton, F., and G. Wilkinson. Sovremennaia neorganicheskaia khimiia, vols. 1–3. Moscow, 1969. (Translated from English.)
Nekrasov, B. V. Osnovy obshchei khimii, vol. 1. Moscow, 1965.
Kislorod, parts 1–2. A handbook edited by D. L. Glizmanenko. Moscow, 1967.
Razdelenie vozdukha metodom glubokogo okhlazhdeniia, vols. 1–2. Edited by V. I. Epifanova and L. S. Aksel’rod. Moscow, 1964.
Spravochnik po fiziko-tekhnicheskim osnovam glubokogo okhlazhdeniia. Moscow-Leningrad, 1963.
oxygen
[′äk·sə·jən] (chemistry)How Many Atoms Are In O2
oxygen
oxygen
Atomic No Of Oxygen Gas
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
Atomic No Of Oxygen
